If you are having a hard time accessing the 3 Definitions Of Acids And Bases page, Our website will help you. Find the right page for you to go to 3 Definitions Of Acids And Bases down below. Our website provides the right place for 3 Definitions Of Acids And Bases.

https://byjus.com/chemistry/acids-and-bases
An acid is any hydrogen containing substance that is capable of donating a proton hydrogen ion to another substance A base is a molecule or ion able to accept a hydrogen ion from an acid Acidic substances are usually identified by their sour taste

https://www.thoughtco.com/acids-and-bases-definitions-603664
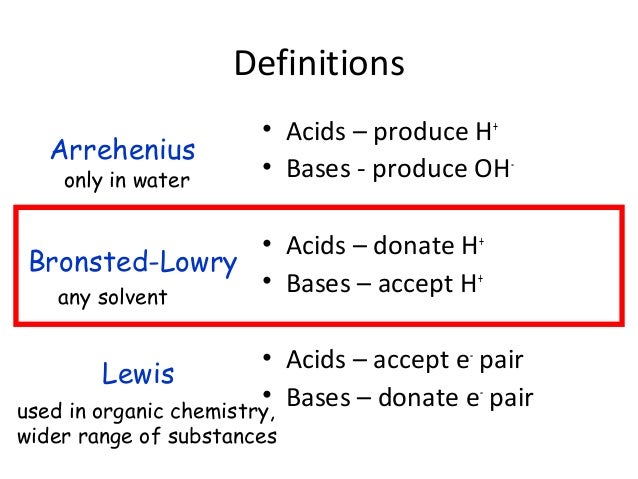
There are several methods of defining acids and bases While these definitions don t contradict each other they do vary in how inclusive they are The most common definitions of acids and bases are Arrhenius acids and bases Br nsted Lowry acids and bases and Lewis acids and bases
https://chem.libretexts.org/Courses/University_of...
Learning Objectives Identify an Arrhenius acid and an Arrhenius base Identify a Br nsted Lowry acid and a Br nsted Lowry base Identify conjugate acid base pairs in an acid base reaction There are three major classifications of substances known as acids or bases The theory developed by Svante Arrhenius in 1883 the Arrhenius
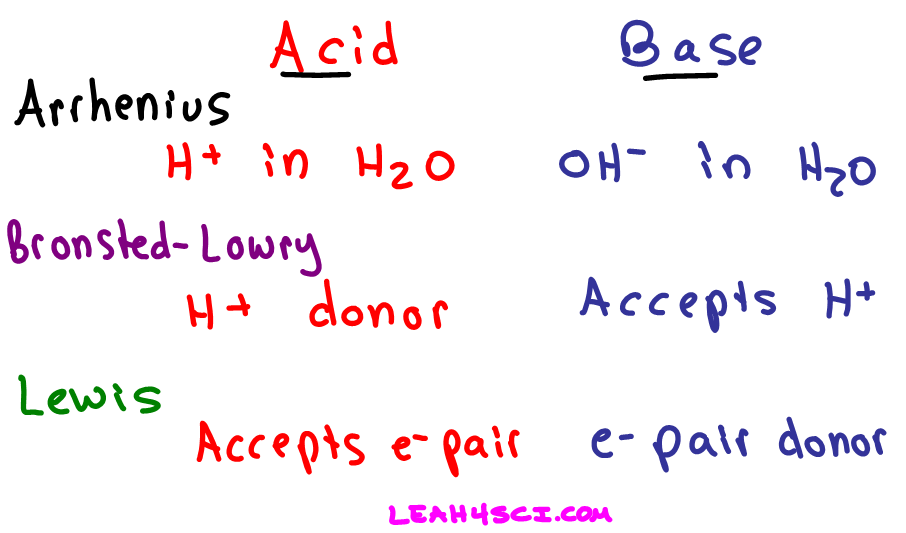
https://chem.libretexts.org/Bookshelves...
Arrhenius s Definition of Acids and Bases The earliest definition of acids and bases is Arrhenius s definition which states that An acid is a substance that forms hydrogen ions H when dissolved in water and A base is a substance that forms hydroxide ions OH when dissolved in water

https://sciencenotes.org/acid-base-chemistry
Acids and Bases Definitions Acids and bases are two types of compounds that readily react with one another Acids are substances that donate protons H ions or accept electron pairs Common examples include vinegar acetic acid CH COOH citrus fruits citric acid C H O and stomach acid hydrochloric acid HCl
https://chemed.chem.purdue.edu/.../ch11/acidbase.php
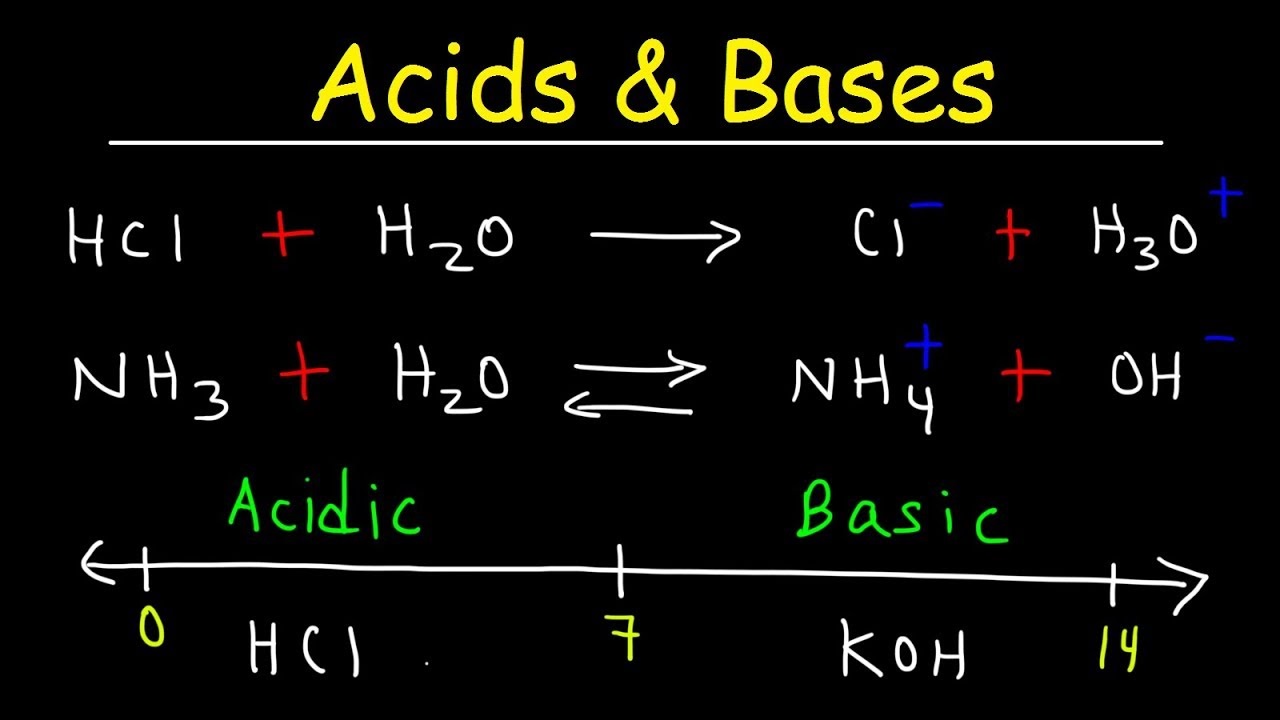
These definitions tie the theory of acids and bases to a simple laboratory test for acids and bases To decide whether a compound is an acid or a base we dissolve it in water and test the solution to see whether the H or OH ion concentration has increased

https://www.osmosis.org/learn/Definitions_of_acids_and_bases
Bases are compounds that release hydroxide ions OH when dissolved in water Acids and bases are classified as strong or weak based on how they dissociate in solutions Strong acids and bases completely dissociate in water releasing all their H or OH ions

https://www.khanacademy.org/science/chemistry/acids-and-bases-topic
Acids bases and solutions Unit 9 Redox reactions and electrochemistry Unit 10 Kinetics Unit 11 Practice for AP Chemistry exam world class education to anyone anywhere Khan Academy is a 501 c 3 nonprofit organization Donate or volunteer today Site Navigation About News Impact Our team Our interns Our content specialists
https://www.varsitytutors.com/high_school...
There are three principle definitions for acids and bases The Arrhenius definition is the simplest and states that acids are compounds that increase proton concentration in solution while bases are compounds that increase hydroxide concentration in solution
Thank you for visiting this page to find the login page of 3 Definitions Of Acids And Bases here. Hope you find what you are looking for!